News
CONTACT US
contact ustime:2023-05-17 Author: Sunny Number of visits:174
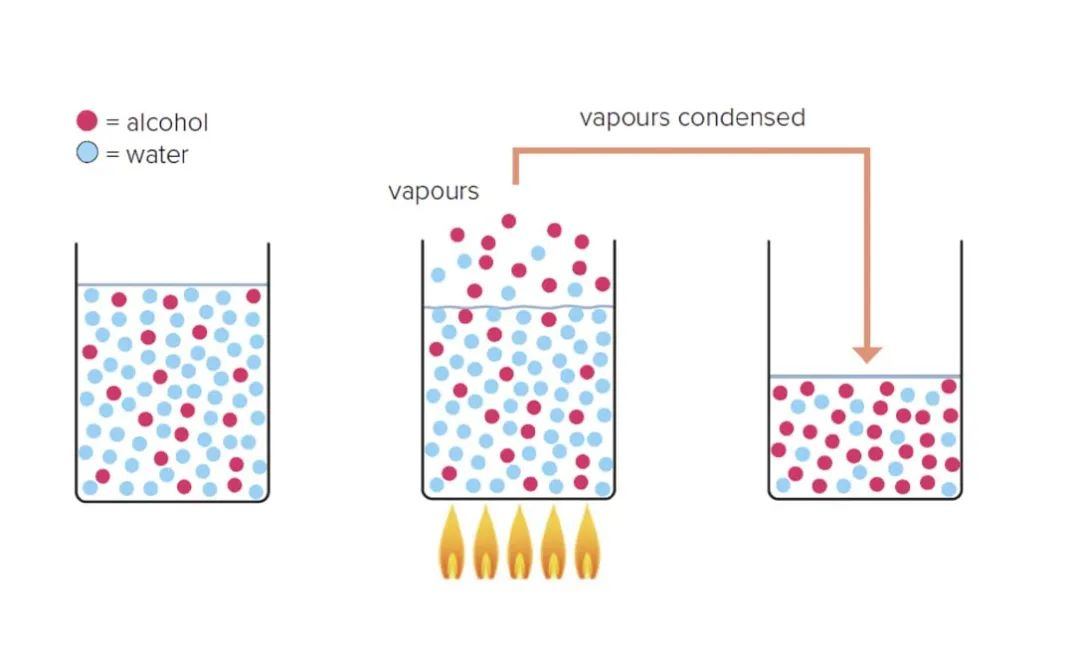
Taking the pot still as an example, since the pot bottom is mostly used for heating, the temperature gets lower and lower from the bottom to the top. As the steam rises, the steam mixed with water and ethanol condenses, releasing heat and turning into liquid droplets.
After the droplets are formed, the steam continues to rise from the bottom up, and the steam contains more heat, so it also exchanges heat with the droplets, creating a "micro-distillation" environment.
As the droplet absorbs heat again, some of it becomes vapor again and continues to rise. At this point, the boiling point of ethanol is low, so more ethanol is released from the droplet. In this way, the micro-distillation of the droplets also increases the alcohol concentration again. Micro-distillation occurs continuously, eventually pushing more alcohol to the top of the still where it is collected, resulting in a higher alcoholic liquor.